In this article, you are going to learn the following subjects. Hopefully, they will help you know more about the emulsion explosives sensitizing agents.
- What is emulsion explosive sensitizing agent?
- How the sensitizing agents influence the emulsion explosive?
- Two main categories of sensitizing agents used in emulsion explosives.
- Sensitizing Agent – Expandable Microspheres
- Sensitizing Agent – Glass Microspheres
- Sensitizing Agent – Perlite
- Sensitizing Agent – Cenospheres
- Sensitizing Agent-Sodium Nitrite
- Comparison of Physical and Chemical Sensitizing Agents
- Comparison of Glass and Polymer Microspheres
What is emulsion explosive sensitizing agents?
Emulsion explosive sensitizing agent refers to a substance or compound added to the explosive formulation to enhance its sensitivity to initiation. Sensitizing agents are carefully selected and dosed to optimize the sensitivity, performance, and safety of emulsion explosives for specific applications. However, it is essential to balance sensitivity with stability and to adhere to stringent safety protocols during the manufacturing, handling, storage, and transportation of sensitized explosive formulations.
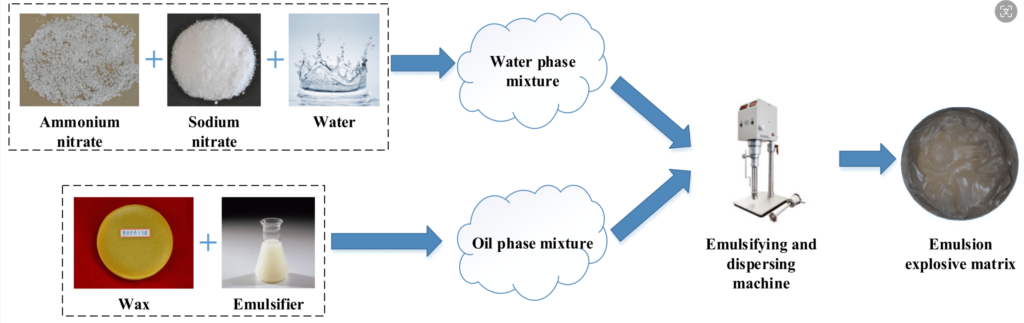
How the sensitizing agents influence the emulsion explosive?
The emulsion matrix without sensitizing agent is not an explosive, as its high density and homogeneity not supporting the detonation processes. The emulsion matrix must be sensitized to become an explosive state and be capable of sustained detonation. Sensitization means adding substances that lower the density and introduce homogeneity “defects” into the matrix, such as hollow structure spheres (gas bubbles), creating hot-spots. The gas bubbles (hot-spots) absorb energy, heating up to high temperatures, allowing the detonation to be sustained.
Two main categories of emulsion explosives sensitizing agents:
1.Physical Sensitizing Agents
Physical sensitizers are substances or additives that alter the physical properties of the explosive matrix to enhance its sensitivity. These sensitizers may introduce voids, gas pockets, or discontinuities within the explosive material, making it more prone to initiation. Examples of physical sensitizers include:
- Microspheres: Hollow spherical particles, such as glass microspheres or expandable microspheres, can be added to emulsion explosives to create voids or gas-filled channels that amplify the shockwave during initiation.
- Porous additives: Porous materials, such as porous granules or fibers, can be incorporated into emulsion explosives to increase their surface area and create pathways for the propagation of the initiation signal.
2.Chemical Sensitizing Agents
Chemical sensitizers are compounds that chemically react with the explosive matrix to increase its sensitivity. These sensitizers may undergo decomposition or chemical reactions upon initiation, releasing gases or heat that amplify the shockwave and promote detonation. Examples of chemical sensitizers include:
- Sensitizing oils: Certain oils, such as nitroglycerin or ethylene glycol dinitrate, can be added to emulsion explosives to increase their sensitivity.
- Nitro compounds: Nitroaromatic compounds, such as nitrobenzene or nitrotoluenes, are commonly used as sensitizers in emulsion explosives.
- Organic peroxides: Peroxide compounds, such as di-tert-butyl peroxide, can act as sensitizers by decomposing rapidly upon initiation, generating heat and gas.
- Sensitizing salts: Certain inorganic salts, such as lead azide or lead styphnate, can be added to emulsion explosives to increase their sensitivity to initiation.
Emulsion Explosives Sensitizing Agents-Expandable Microspheres
可膨胀微球是一种微米级的聚合物颗粒,由聚合物外壳和包裹的烷烃气体组成。当加热时,聚合物外壳软化,同时内部气体压力增加,从而形成体积的显著膨胀。
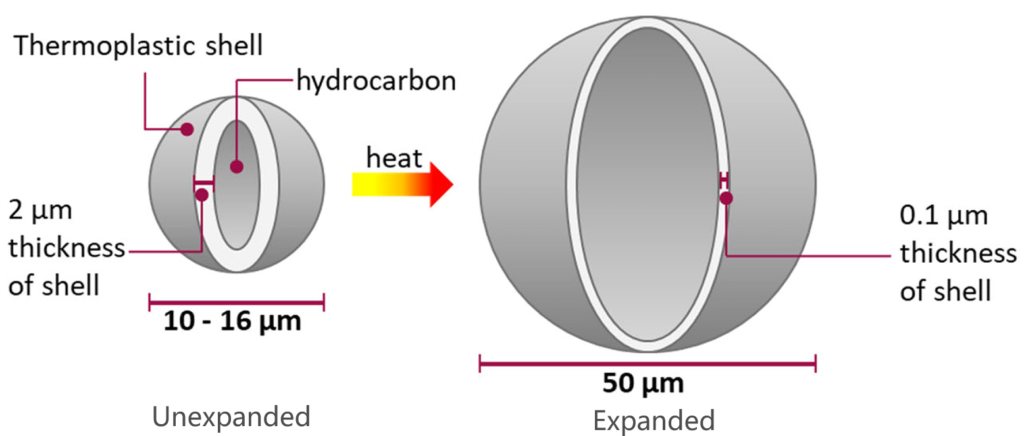
Compressibility
Expanded microspheres show spherical shape when it is under atmospheric pressure.
Expanded microspheres will be compressed under high pressure, for example 5 bar.
Expanded microspheres will be back to original spherical shape when pressure is released.
The resilience will prevent the microspheres from breaking during filling into explosives cartridges.
Closed-Cell Structure
Expandable microspheres has the uniform and controlled closed cell structure.
Extremely Low Density
To get the same density reduction, expanded microspheres will take much less dosage compared to the glass bead, saving you much on total cost.
Emulsion Explosives Sensitizing Agents-Glass Microspheres
Glass Microspheres is a kind of hollow microspheres, ultralight inorganic nonmetallic powder materials. It’s micron-size and looks like foam bubbles with the microscope, also known as glass bubble. It’s a good alternative filler instead of conventional fillers, such as talc, calcium carbonate, silicas, etc.
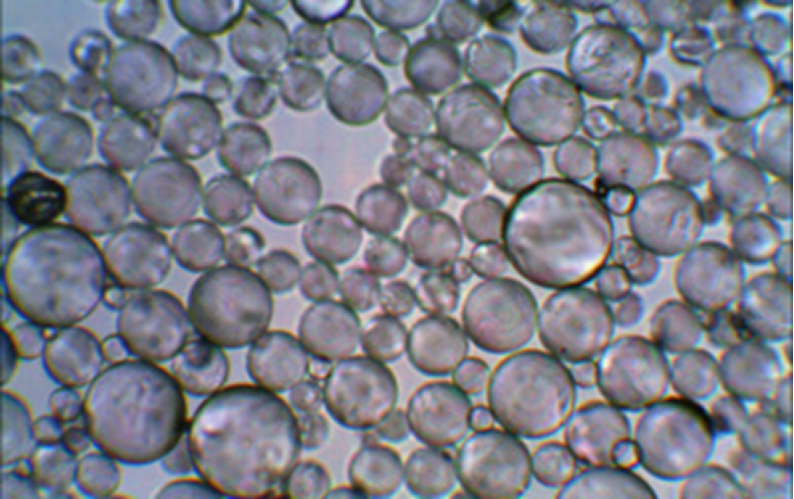
Advantages of Hollowlite Hollow Glass Microspheres
The ultra low density of glass microsphere are mainly used to reduce the part weight or compound material weight.
- Pure white color: Hollow Glass Microsphere can be widely used in products which have high requirements for looks and colors.
- Low density, reducing the products’ basic weight obviously after filling.
- High loading volume, which can substitute and save more resins, reducing cost.
- High dispersion and good fluidity.
- Dimensional stability, reduced warpage and shrinkage.
- Heat insulation, sound insulation, mostly used as heat insulation paints and coatings, automotive sealants.
- Corrosion resistant, fire resistant, non-conducting.
- Stable chemical properties.
Emulsion Explosives Sensitizing Agents-Perlite
Perlite is a lightweight granular material that’s white in colour. It looks and feels like little bits of polystyrene but is actually made from expanded volcanic glass, heated to 1000°C until it ‘pops’ (like popcorn) to many times its original size. It’s lightweight, sterile, and easy to handle, and is long-lasting. It’s neither alkaline nor acidic.

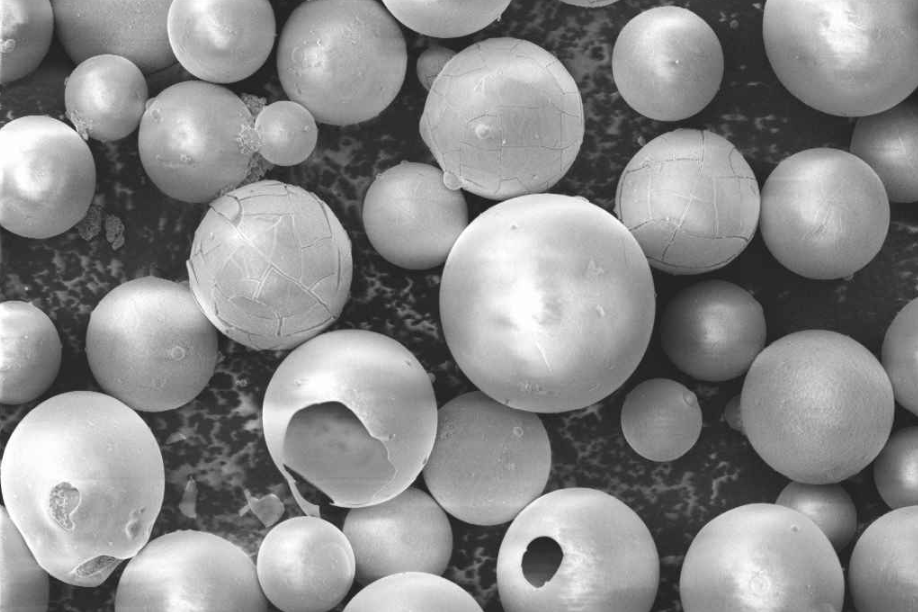
Emulsion Explosives Sensitizing Agents-Cenospheres
Cenospheres are a unique byproduct of coal combustion, typically generated during the burning of coal in thermal power plants. These hollow, lightweight particles are made up of silica, alumina, and iron oxide, and they possess a number of fascinating properties that make them valuable in a variety of industrial applications. The spheres have high crush strength, pressure resistance, excellent thermal insulation properties, are non-toxic and non-hazardous. The particle shape allows for an easier application and provides a smoother finish.
Emulsion Explosives Sensitizing Agents-Sodium Nitrite
Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.

Aqueous solutions of sodium nitrite are primarily used as chemical sensitising agents. The use of sodium nitrite is based on a reaction between sodium nitrite and acidified ammonium nitrate in the presence of thiourea. This reaction produces nitrogen which, in the form of micro bubbles, fills the entire volume of the emulsion, reducing the density and creating hot spots. The rate of this reaction is strictly dependent on the temperature of the components and the reaction continues until one of the reagents is depleted. Chemical sensitisation can be problematic in cold environments, sometimes necessitating additional acidification, in order to achieve the desirable rate of the sensitisation reaction
Comparison of Physical and Chemical Sensitizing Agents
Physical sensitization are mainly used in producing cartridge emulsion explosives. Chemical sensitization is mainly applied in bulk emulsion explosives. It depends on the reaction of the sensitizing agent with oxidizing agents in the emulsion explosives matrix, as this reaction results in the evolution of gas that produces small bubbles across the entire volume of the sensitized matrix. Both sensitization results in the density reduction of the emulsion explosives matrix.
It is very important that the diameter of the gas pores created by the sensitizing agent greatly impacts the detonation velocity and critical diameter of the emulsion explosives. The control of the dimensions and distribution of gas pores will be the key factor to develop new emulsion explosives sensitization methods. Physical sensitization could introduce a very uniform pore size as microspheres can be produced to have a narrow size distribution. For chemical sensitization, the size of the gas pores depends on the type of sensitizing agent, sensitization time, temperature and viscosity of emulsion explosive matrix .
Comparison of Glass and Polymer Microspheres
- Density/Volume: Expanded microspheres has 5-10 times bigger volume than the glass spheres;
- Compressibility: Expanded microspheres has good compressibility and could withstand high shear force without any breakage of spheres. The glass spheres is rigid shell and need to disperse in slow stirring, or the spheres will break easily and lose the volume.
- Cost Saving: expanded microspheres has low cost in final product than the glass spheres to get the same density reduction.

For more applications, please check our application sections:
https://www.expandablemicrosphere.com/applications
更多有趣的视频,请查看我们的 Youtube 频道: